Background risk:
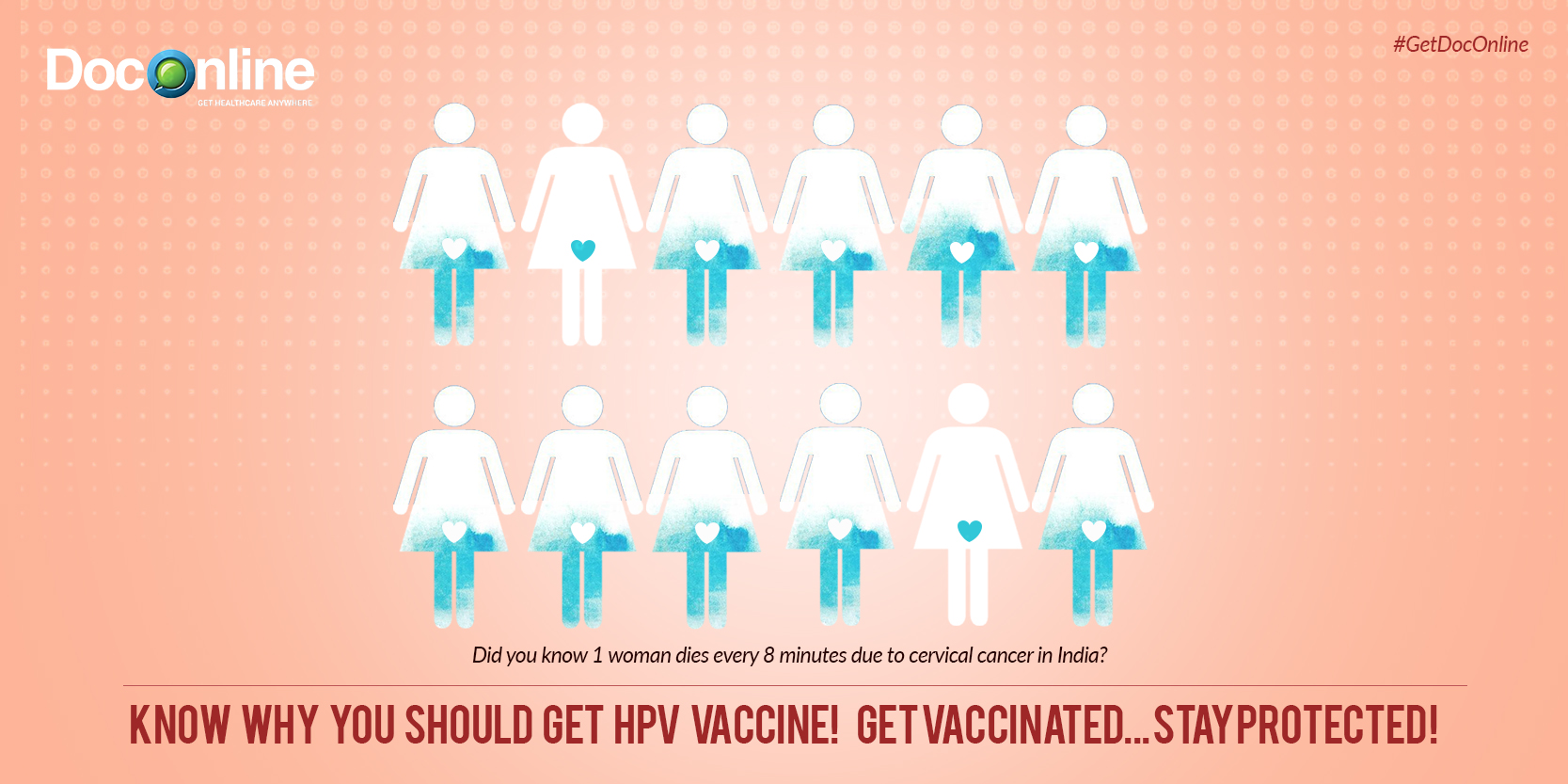
Cervical cancer is the second most common cancer among women in India and the fourth most common worldwide. It has been roughly estimated that one woman dies every 8 minutes due to cervical cancer in India.
Infection by the human papillomavirus (HPV), which is the most common sexually transmitted viral infection is the most important risk factor for cervical cancer. HPV infection is highly influenced by age and sexual activity. It is believed that almost 75% of adults are infected by at least one type of HPV. However, not all women with an HPV infection will develop cervical cancer. In majority of people with healthy immune system, the body can spontaneously clear the infection. Sometimes, in a very small proportion of women, the infection does not go away and remains persistent. This chronic infection, especially by a high-risk subset of human papilloma virus (HPV), can cause precancerous changes in cells and ultimately progress to cervical cancer.
Prophylactic Vaccines:
Mortality and morbidity due to cervical cancer could be reduced by a holistic approach that includes prevention, early diagnosis, effective screening and treatment.
Vaccines were developed targeting HPV types 16 and 18, the most common oncogenic types of HPV, which are together responsible for approximately 70% of cervical cancer cases. At present, three prophylactic HPV vaccines, namely Gardasil, a quadrivalent vaccine (HPV types 16/18), Gardasil 9, a nonavalent vaccine (6/11/16/18/31/33/45/52/58) and Cervarix, a bivalent vaccine (HPV types 6/11/16/18), which have been prepared from purified L1 structural proteins by recombinant technology. All the available vaccines are equally efficacious and safe.
Dosage and schedule:
The recommended age for initiation of vaccination is 9-14 years. Ideally, patients should be vaccinated before onset of sexual activity. As of current regulations in India, catch up vaccination is permitted up to the age of 45 years, although the efficacy of the vaccine seems to wane with increase in age.
The vaccines have been prepared from purified L1 structural proteins by recombinant technology given at a dose of 0.5 ml injected intramuscularly. A total of three doses at 0, 2 and 6 months are recommended with Gardasil 9 and Gardasil or 0, 1 and 6 months with Cervarix.
However currently ,a two dose schedule is recommended for females < 15 years at 0 and 6 months.
Catch up schedule:
If the HPV vaccine schedule is interrupted, the vaccine series need not to be restarted. If the series is interrupted after the first dose, the second dose should be administered as soon as possible, with an interval of at least 12 weeks between the second and third doses. If only the third dose is delayed, it should be administered as soon as possible.
Vaccination in pregnancy:
HPV Vaccine is ideally not recommended for pregnant women, but if the vaccine is unknowingly administered during pregnancy, it is not necessary to become excessively worried or to consider termination of pregnancy; however, the remaining doses should be delayed until completion of the pregnancy.
Vaccination impact on Screening:
Even though vaccination at the right age offers protection against the most common cervical cancer-causing HPV viruses, screening is still necessary to pick up abnormalities caused by other HPV virus subtypes that could lead to cervical cancer. Hence, screening programs should continue as per recommendations. Vaccination and screening may together lead to a substantial reduction in the risk of cervical cancer. To know more about cervical cancer and its prevention, one can easily consult a doctor online at one's convenience.
Get Vaccinated....Stay Protected!!!